What are Atoms? – Hello Sinaumed’s friends, When you were in 10th grade high school, you will get information about atomic theory, such as understanding, types and other aspects related to atomic theory and models. also understand the development of atomic theory in this world.
Especially for those of you who are majoring in chemistry, you will find courses that explain atomic matter. Thus, not all students will be able to re-learn this material. For those of you who later decide to become lecturers or teachers, there is the possibility of learning information about atoms.
Specifically used as a reference in the preparation of materials or teaching materials. Because if you only rely on memory, compiling teaching materials is not enough. However, it must also be supported by relevant and qualified sources. Because it could be a lot of science or material theory of atoms that have been developed.
Basically every object in this world must have a very small part, so if that small part is divided again, there will be even smaller parts. Well, this smallest fraction is called an atom.
The atom itself can be considered as the smallest element of all existing matter. In a matter of atoms can be explained like this. Especially in the scientific world, the atom is experiencing development. Every development in the atom is the result of an evaluation of the previous atomic theory.
Want a more complete explanation about the meaning of atoms and their history and want to know the atomic components? Don’t hesitate anymore, let’s read this article right away, then you will know the meaning and history and components of the atom.
Understanding Atoms
The atom is a basic unit of matter consisting of an atomic nucleus and a cloud of negatively charged electrons that surrounds it. The atomic nucleus consists of positively charged protons and neutrally charged neutrons (except in the hydrogen-1 atomic nucleus, which has no neutrons). The electrons in an atom are bound in the atomic nucleus by electromagnetic forces.
A collection of such atoms can also bond with each other and form a molecule. Atoms containing the same number of protons and electrons are neutral, while those containing an asynchronous number of protons and electrons are either positive or negative and are claimed to be ions. Atoms are grouped according to the number of protons and neutrons that are still present in the atomic nucleus. The number of protons in an atom determines the chemical element of that atom and the number of neutrons determines the isotope of that element.
The Greek word for an atom is (ἄτομος/átomos, α-τεμνω) , which means something that cannot be cut or divided. The concept of the atom as an indivisible component was first proposed by Indian & Greek philosophers. In the 17th and 18th centuries, chemists laid down the foundations of this idea using and demonstrating that specific substances could not be further broken down using chemical methods.
During the late 19th and early 20th centuries, physicists discovered the structure and subatomic components within the atom, demonstrating that the ‘atom’ was indivisible. The principles of quantum mechanics were used by physicists who succeeded in modeling the atom.
In everyday observations, it is relatively believed that the atom is a very small object that has a proportionally small mass. Atoms can only be monitored using specific tools such as an atomic force microscope. Over 99.9% of an atom’s mass is centered in the atomic nucleus, with protons and neutrons of nearly the same mass. Every element has at least one isotope with an unstable nucleus, which can undergo radioactive decay.
This can cause transmutation, which changes the number of protons and neutrons in the nucleus. Electrons bound in atoms contain a number of energy levels, or orbitals, that are stable and can undergo transitions between these levels and absorb or emit photons synchronously with the energy disparity between the levels. The electrons in an atom determine the chemical properties of an element, and influence the magnetic properties of that atom.
Understanding Atom According to Experts
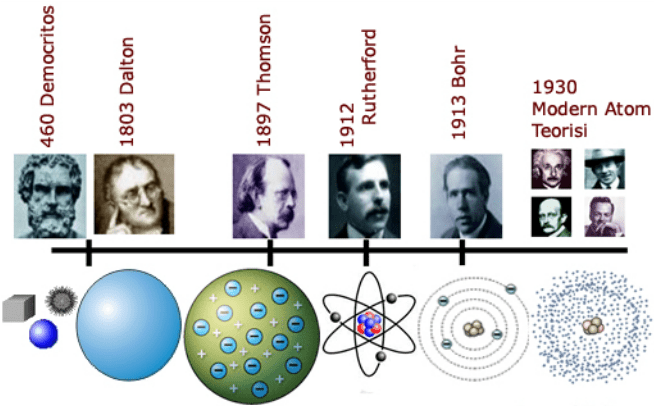
1. John Dalton
Atoms are the smallest particles of a substance that cannot be broken down into smaller particles by normal chemical reactions.
2. Leucippus and Democritus
Atom is the smallest part of matter that cannot be divided into certain parts. Atoms are the building blocks of all matter in this world.
3.Joseph John Thomson
Atoms are positively charged balls surrounded by electrons like raisins.
4. Ernest Rutherford
Atoms are particles consisting of neutrons and protons and surrounded by electrons.
Whereas in the Big Indonesian Dictionary (KBBI), atom means the smallest chemical element (after the nucleus) that can stand alone and combine with other elements. Not only that, from the four definitions of atoms put forward, atoms can be understood as the smallest and inseparable particles.
“The smallest and indivisible particle” , this thought comes from Democritus. He was the first scientist to reveal it. Atomic thoughts or opinions are not scientific research, but only Democritus thoughts.
From the thoughts of Democritus, many scientists are challenged to do research on atoms. In fact, until now, atomic research is still ongoing.
In general, atoms are circular in shape with diameters ranging from 6 to 30 mm. The electromagnetic force that exists in atoms can bind particles such as protons, neutrons, and electrons. Atomic bonds with protons, neutrons, and electrons make these atoms and particles form molecules. So far, atoms have not been seen with various technological tools.
The atomic nucleus is often called the daughter nucleus. An atom has a nucleus surrounded by electrons, protons and neutrons. Electrons carry a negative charge. On the other hand, the proton is positive. After having a positive and negative charge, a neutron can be said to have a charge that is not useful to anyone, simply that a neutron is neutral.
A Brief History of the Atom
The idea that matter is made up of indivisible units has been around for a millennium. However, these thoughts remain abstract and philosophical, not based on empirical observations and experiences.
Philosophically, descriptions of the nature of the atom vary according to culture and philosophical school, and often contain spiritual elements. However, the basic idea of the atom was accepted by scientists thousands of years ago because it could elegantly explain new discoveries in chemistry.
The first reference to the concept of the atom comes from ancient India in 800 BC, it is described in Jainist philosophical texts such as this and the like. The Nyaya and Vaisesika schools developed theories explaining how atoms combine to form more complex objects. A century later, Leucippos referred to the atom in the West, which was later codified by his student Democritus
Around 450 BC, Democritus coined the term átomos (Greek: ἄτομος) , meaning “uncut” or “undividable”. Democritus’ atomic theory was not an attempt to describe physical phenomena in detail, but a philosophy that attempted to provide answers to the changes that occurred in nature.
Similar philosophy also exists in India, however, modern science has decided to use the term “atom” coined by Democritus. Democritus also said that the atoms in water are so slippery that water can flow everywhere while the atoms in salt are covered with sharp thorns which give a salty taste to the tongue.
Further advances in the understanding of atoms began with developments in chemistry. In 1661, Robert Boyle published The Skeptical Chymist , who argued that the world’s matter was composed of various combinations of “corpuscles”, that is, different atoms.
This contrasts with the classical view that matter is composed of the elements air, earth, fire and water. In 1789, the term element or element was defined by Antoine Lavoisier, a French aristocrat and researcher, as a basic substance that cannot be divided by chemical methods.
Aristotle said that there are 4 basic elements in the earth and when they are all combined they will form the compounds we see. At that time his student asked: “Can we make gold if we combine all the basic elements earlier?” Aristotle answered “Yes” .
It made scientists wonder where it was 200 years later. In 1669, German chemist Hennig Brand distilled 60 buckets of urine because he thought there was real gold in it (because urine is golden yellow) and as a result his chemical apparatus glowed in the dark.
He named him Phosphorus (Phosphorus) after the Greek word “Phosphoros” which means evening star. He was the first person in the era of AD, which was previously the discovery of arsenic 300 BC.
In 1803, John Dalton used the concept of atoms to explain why elements always react in spherical and fixed proportions, and why some gases are more soluble in water than others. . He proposed that each element contains a single, unique atom, and these atoms can then combine to form chemical compounds.
This particle theory was confirmed in 1827, when the botanist Robert Brown used a microscope to observe dust floating on water and found that it moved randomly.
This phenomenon is known as “Brownian movement”. In 1877 J Desaulx proposed that this phenomenon was caused by the thermal motion of water molecules, and in 1905 Albert Einstein carried out a mathematical analysis of this motion. French physicist Jean Perrin later used Einstein’s work to experimentally determine the mass and size of an atom, which would become the final verification of Dalton’s atomic theory.
Based on his work on cathode rays, JJ Thomson in 1897 discovered the electron and its subatomic properties. This destroys the concept of the atom as an indivisible unit. Thomson believed that electrons were evenly distributed throughout the atom and that the charges were balanced by the presence of a sea of positive charge (the plum pudding model).
Atomic Components
1. Subatomic Particles
Although originally the term atom meant a particle that could not be chopped up or further reduced into smaller particles, in modern scientific terminology, atoms are composed of a large number of subatomic particles. The particles that make up the atom are electrons, protons and neutrons. But hydrogen-1 has no neutrons. The same is true for the positive hydrogen ion H+.
Of these subatomic particles, the electron is the lightest, has a mass of 9.11 × 10−31 kg and has a negative charge. The size of the electron is so small that there is no measuring technique that can be used to measure its size. The proton has a positive charge and a mass 1,836 times that of the electron (1.6726 × 10−27 kg). Neutrons have no electrical charge and have a free mass 1,839 times that of electrons or (1.6929 × 10−27 kg).
In the standard example of physics, both protons and neutrons are composed of elementary particles called quarks. Quarks belong to the class of fermion particles which are one of two basic building blocks (the other being leptons). There are six types of quarks and each has a fractional electric charge of +2/3 or −1/3. Protons are made up of two up and one down quarks, while neutrons are made up of one up and 2 down quarks.
This difference in the composition of the quarks affects the mass and charge disparity between the two particles. The quarks are held together by a powerful nuclear force mediated by the gluons. Gluons are members of the benchmark bosons which are mediators of physical forces.
2. Atomic Nucleus
The atomic nucleus consists of protons and neutrons bound together at the center of the atom. Collectively, these protons and neutrons are called nucleons (particles that make up the nucleus). The diameter of the atomic nucleus varies from 10−15 to 10−14 m. The approximate radius of the nucleus is 1.07 fm, where A is the number of nucleons.
This is very small compared to the atomic radius. The nucleons are held together by a potential gravitational force called the strong residual force. At distances less than 2.5 fm, these forces are stronger than the electrostatic forces that cause the protons to repel each other.
Atoms of the same chemical element have the same number of protons, which is called the atomic number. The nucleus of an atom with a specific atomic number, specific mass number, and half-life is called a nucleotide. An element can have a varying number of neutrons. These variations are called isotopes.
Isotopes are elements with the same mass number but different atomic numbers. Isotopes of elements with the same number of electrons but different atomic numbers and masses. The number of protons and neutrons in an atom will determine the atomic nuclide, while the number of neutrons relative to the number of protons will determine the stability of the atomic nucleus, with isotopes of a particular element undergoing radioactive decay.
Neutrons and protons are two different types of fermions. The Pauli exclusion principle prohibits the existence of identical fermions (such as many protons) occupying the same quantum physical state at the same time.
Therefore, each proton in the atomic nucleus must occupy a different quantum state with its own energy level. The Pauli principle also applies to neutrons. This prohibition does not apply to protons and neutrons which have the same quantum state.
For atoms with low atomic numbers, atomic nuclei with more protons than neutrons tend to fall to a lower energy level through radioactive decay, leading to an equilibrium of protons and neutrons. Therefore, an atom with a balanced number of protons and neutrons is more stable and less likely to decay.
However, as the number of atoms increases, the repulsion between protons causes the atomic nucleus to require a higher proportion of neutrons to maintain its stability. In the heaviest nuclei, the neutron/proton ratio needed to maintain stability increases to 1.5.
3. Electron Cloud
Electrons in an atom are attracted by protons in the atomic nucleus through electromagnetic forces. This force holds the electrons in the wells of the electrostatic potential around the nucleus. This means that an external force is required for electrons to escape from atoms. The closer an electron is to the nucleus, the greater the force of attraction, as a result, electrons that are close to the center of the potential well require greater energy to escape.
Electrons, like other particles, have properties such as particles as well as waves (wave-particle dualism). The electron cloud is a region in the potential well where each electron makes a homogeneous 3-dimensional stationary wave (i.e. a wave that is not moving relative to the nucleus).
This behavior is influenced by the atomic orbital, which is a mathematical function that calculates the probability that an electron will be in a particular location when its position is measured. There will only be a specific set of orbitals around the core, as other wave patterns will rapidly decay to more stable forms.
Each atomic orbital corresponds to a specific electron energy level. Electrons can change their state to a higher energy level by absorbing a photon. As well as being able to rise to a higher energy level, an electron can also descend to a lower energy state by radiating the excess energy into a photon.
The energy required to remove or add an electron (electron binding energy) is less than the binding energy for nucleons. For example, it takes only 13.6 eV to remove an electron from a hydrogen atom. Compare that to the 2.3 MeV required to split a deuterium nucleus.
Atoms are electrically neutral because they have the same number of protons and electrons. Atoms that lack or gain electrons are claimed to be ions. Electrons that are located outside of the nucleus can be transferred or shared with other nearby atoms. In this way, atoms can bond together to create molecules.