Definition and function of universal indicators – In measuring pH many methods are used by researchers in conducting research. One method that is often used is universal indicators. This indicator is often used in research because it is easier to use than other methods. In addition, the results obtained are also accurate and fast.
However, for ordinary people, students and students who are new to pH measurement methods will find it difficult to understand what the meaning and function of universal indicators are. Moreover, how to use the universal indicator itself. So to anticipate this, here is a complete explanation of universal indicators.
What is Universal Indicators?
Universal indicator is a paper that has various colors that can be used to measure a pH value in a solution. Generally, the colors owned by universal indicator paper are 4 colors or more. Each paper that is inserted into the solution can change color according to the level of the pH value of the solution.
This universal indicator method usually takes advantage of the color change on the paper that occurs due to a comfort in a liquid solution to measure how high the pH is in the solution.

Universal Indicator Function
After knowing the meaning, let’s move on to the function of this universal indicator. Each research method has its own function in examining an object. Likewise, the universal indicator research method has the function of measuring several levels of compounds in a liquid solution. This method is very easy to use in research so that many researchers and students use this method to measure a pH level in a compound.
This universal indicator function is obtained from paper that has 5 colors. Each color that will be produced by universal indicator paper has various colors, namely:
| pH range | Description | Colour |
|---|---|---|
| < 3 | Strong acid | Red |
| 3–6 | Weak acid | Orange or Yellow |
| 7 | Neutral | Green |
| 8–11 | Weak alkali | Blue |
| > 11 | Strong alkali | Indigo or Violet |
The colours from yellow to red indicate an acidic solution, colours blue to violet indicate an alkaline solution and a green colour indicates that a solution is neutral.
| Indicator | Low pH colour | Transition pH range | High pH colour |
|---|---|---|---|
| Thymol blue (first transition) | Red | 1.2 – 2.8 | Yellow |
| Methyl orange | Red | 3.2 – 4.4 | Yellow |
| Methyl red | Red | 4.8 – 6.0 | Yellow |
| Bromothymol blue | Yellow | 6.0 – 7.6 | Blue |
| Thymol blue (second transition) | Yellow | 8.0 – 9.6 | Blue |
| Phenolphthalein | Colourless | 8.3 – 10.0 | Fuchsia |
Wide-range pH test papers with distinct colours for each pH from 1 to 14 are also available. Colour matching charts are supplied with the specific test strips purchased.
How to Use Universal Indicator
Universal indicators is very easy to use . There is no need to need many tools in using universal indicator methods in a study.
Researchers only need to buy research indicator paper and prepare a liquid solution that will be used in research and follow these steps:
1. Take Universal Indicator Paper
The first step is that the universal indicator paper must be taken with care and try not to touch the dye contained in this universal indicator paper. Because if the dye contained in this paper is contaminated with other substances that have acidic or alkaline properties, this indicator paper can be damaged.
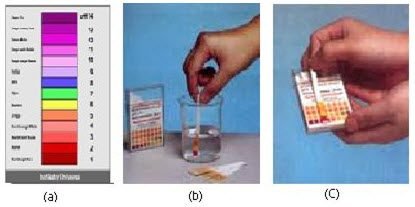
2. Put In Liquid Solution
The second step is the universal indicator paper is inserted into the beaker which already contains the liquid solution. Make sure all parts of the universal indicator paper have been immersed in the liquid solution. Wait a few minutes to make sure that all the substances in the liquid have adhered to the universal indicator paper and changed color.
Then, remove the universal indicator paper and let it sit for 4-5 minutes until it dries completely. Make sure the paper is dry properly. If not, the paper will change color again so that the results of the study will change.
3. Compare Using Scale
The use of a scale in comparing the levels of substances contained in universal paper is something that cannot be missed. Because this scale will be used to measure the levels of substances contained in a liquid solution. In general, the scale used by researchers to measure color change is between pH 0-4.
The method used in determining the concentration of the solute is by matching the color of the indicator paper that has been inserted into the liquid solution with a universal indicator scale. After determining the indicator scale that is considered the most suitable for the color of the universal indicator paper. Then the researcher will know the pH levels contained in the liquid solution that has been tested.
In addition to studying the meaning and function of universal indicators . A researcher, student or student who wants to conduct research using the universal indicator method must also understand the advantages and disadvantages of a method itself. These advantages and disadvantages can be used in considering a research method to be taken.
Disadvantages and Advantages of Universal Indicators
It is the same with pH research methods or other acid and base measurements . Universal indicators also have their own advantages and disadvantages. There are many advantages possessed by universal indicators and there are also many disadvantages possessed by the pH research method using universal indicators. The following are the advantages and disadvantages of universal indicators:
Advantages of Universal Indicator
1. Easy to Use
The first advantage of the pH measurement method using universal indicators is that it is easy to use. The use of this measurement method can be done anywhere and by anyone. You don’t have to use complicated formulas and so many tools if you want to use universal indicator methods in research.
Usually, this research method has been applied by teachers who teach in secondary schools. So that students can already feel how to do a research that is usually done by expert researchers. This easy use is the main reason for teachers to choose this method in practical activities.
2. Low Price
The price for getting universal indicator paper is relatively cheap compared to other research tools. Thus, many researchers and teachers take advantage of this opportunity to conduct research. In addition, this universal indicator paper is easy to get anywhere, both in big cities and small areas.
The selling price of universal indicator paper which is relatively cheap makes students, students and researchers conduct research on a solution level using universal indicator paper. Research using this method clearly does not require expensive costs.
Lack of universal indicators
1. Not Very Accurate
The first drawback of universal indicator paper is that the results of this method in measuring pH are not 100% accurate. This happens because, this research can be done by everyone who does not necessarily understand correctly the results of a study.
If you want the results of the universal indicator method to be more accurate, it is advisable to take measurements with people who are experts in research, namely a laboratory assistant. Laborers will be more careful in inferring the results from changes in the color of the universal indicator paper and compare the indicator scales better.
Scale limitations
the scale used to match the color of the indicator paper has only the numbers 0-14. So that the level of pH solution that has a level that requires a comma will not be detected. Of course this is very different if you use a more modern digital pH meter . Each concentration of solution does not have a definite number of scales so that further research is needed.
Research using other tools to accompany the universal indicator method is very necessary. It aims to see more accurately the results of the solution under study whether it is one hundred percent accurate or not when using the universal indicator research method.
Well, this article on the meaning and function of universal indicators is written very easily. Hopefully the readers can understand the meaning and function of universal indicators better. So that it can add insight to the readers.


