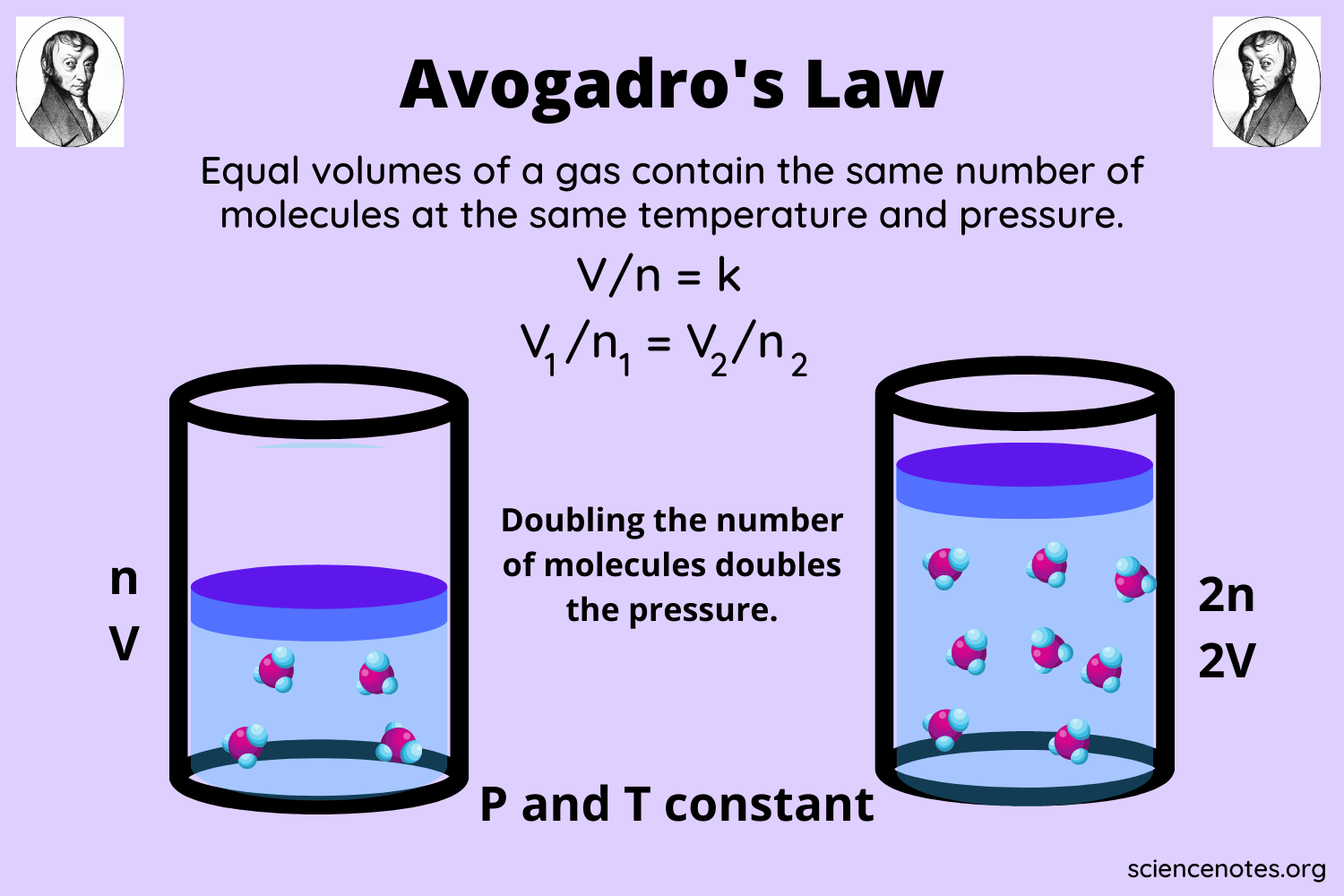Avogadro’s Law and 4 Other Basic Chemical Laws You Must Know – For Sinaumed who are interested in chemistry, this article will be a friend in exploring more profound knowledge about chemistry. The birth of chemistry will always be remembered by scientists who have dedicated their lives to conducting research for years to conduct research.
Chemists such as Avogadro, Lavoisier, Proust, and many other figures.
The basic laws of chemistry are the theories that form the basis of chemical calculations and the quantitative relationships of reactions and products in chemical equations. The following are the five fundamental laws of chemistry, which Sinaumed must know!
1. Avogadro’s law
When studying the substance chapter, there is a fundamental law commonly called Avogadro’s law. Avogadro’s law is a theoretical law that deals with the kinetics of gases. To learn more about the inventor and the law’s sound, look at the following article!
Understanding of Avogadro’s Law
Avogadro’s law was put forward by a physicist from Italy, Lorenzo Romano Amedeo Caro Avogadro, better known as Amedeo Avogadro.
Avogadro’s law was put forward in 1811. Disadue to the Encyclopedia Britannica, Avogadro’s law states that there are empirical relationships in gas theory that apply to all natural gases at specific low pressures and temperatures.
Avogadro’s law reads:
“All gases with the same number of volumes under the same pressure and temperature have the same number of particles or molecules.”
For example, chlorine gas and nitrogen gas, which have a volume of 1 liter and are at standard temperature and pressure, have the same number of particles and molecules.
Adapted from Chemistry LibreTexts, according to Avogadro’s law, the volume of a gas is directly proportional to the number of gas particles or moles when the temperature and pressure are maintained or constant.
Avogadro’s Law Equation
Mathematically, Avogadro’s law can be expressed by the formula:
V = k x n
With V: gas volume (m³)
n: the number of moles of gas (mol)
k: proportionality constant
Meanwhile, the relationship between two gases at the same temperature and pressure, according to Avogadro’s law, is as follows:
With, V1: volume of gas 1 (m³) V2: volume of gas 2 (m³) n1: number of moles of gas 1 (mol) n2: number of moles of gas 2 (mol)
Avogadro’s number
Meanwhile, the number of gas particles depends on the number of moles and Avogadro’s number. Adapted from Lumen Learning, Avogadro’s number is the number of elementary particles, namely molecules, atoms, and compounds per mole of a substance.
Avogadro’s number is a constant that has a value of. The number of particles of a gas can be calculated based on the number of moles and Avogadro’s number.
The formula for the number of particles of a gas is:
X = n x L
With X: the number of gas particles
n: the number of moles of gas
L: Avogadro’s number
Examples of Avogadro’s Law Questions
A 5-liter cylinder contains 2 × 1022 molecules of carbon dioxide gas. How many nitrogen gas molecules are in a 4-liter cylinder at the same pressure and temperature?
Answer:
N1 V1 2×1022
5N2 = N2
V2= N24 = 1,6 x 1022 moles
2. Lavoisier’s law
The law of the conservation of mass in Lavoisier’s science is also called the law of the protection of group. What is the law of conservation of mass? Come on, look at the following meaning.
Understanding Lavoisier’s Law
A French chemist, Antoine Laurent Lavoisier (1743-1794), is the discoverer of the Law of Conservation of Mass. Lavoisier examined a substance’s mass (weight) before and after the reaction. Lavoisier successfully stated the law of conservation of mass in 1789.
Because of these discoveries, Lavoisier is now known as the father of modern chemistry. Previously, Mikhail Lomonosov (1748) had proposed a similar idea and had proven the results in an experiment. However, the law of the conservation of mass presented by Mikhail Lomonosov still tends to be challenging to understand because of the bouncing forces in the earth’s atmosphere.
According to Lavoisier’s experimental results, the amount of substance present before and after a reaction will always be the same if it is in a closed system. Even so, material changes generally occur in an open system, so if a reaction product leaves the system or a substance from the bound environment, the mass of the essence before and after the reaction will be different.
The conclusion that Lavoisier draws regarding the law of the conservation of mass is:
“The mass of the substance before and after the reaction is constant.”
From the sound of the law, it can be concluded that the definition of the law of the conservation of mass is a law that states that the group of a closed system will always be constant or remain the same even though various processes occur in the system.
Lavoisier’s Law Experiment
In experimenting on the law of conservation of mass, Antoine Laurent Lavoisier experimented by heating mercury oxide (HgO) to produce metal mercury (Hg) and oxygen gas (O2), which gave rise to the reaction or Lavoisier’s law formula, which is as follows:
2HgO(l)+O2(g)→2Hg(s)+2O2(g)
Furthermore, the two products will be reacted again to form mercuric oxide. This study shows that the mass of oxygen gas produced by burning mercury oxide is the same as the mass of oxygen needed to convert mercury metal into mercury oxide.
Problems example
1. 5 grams of magnesium metal is reacted with 5 grams of oxygen to form an oxide compound. From this reaction, what mass of magnesium oxide is produced?
Answer:
Pre-reaction mass = post-reaction mass
mass of magnesium metal + mass of oxygen = mass of magnesium oxide
5 grams by mass of magnesium metal + 5 grams by mass of oxygen = 10 grams by mass of magnesium oxide
2. A magnesium metal having a mass of 6 grams is then reacted with oxygen to form 8 grams of oxide compounds. What is the group of the reacting magnesium?
Answer:
Pre-reaction mass = post-reaction mass
Mass of magnesium metal + mass of oxygen = mass of magnesium oxide
6 grams by mass of magnesium metal + mass of oxygen = 8 grams by mass of magnesium oxide
Mass of oxygen = 8 grams by mass of magnesium oxide – 6 grams by mass of magnesium metal
The mass of oxygen = 2 grams
3. Proust’s law
Has Sinaumed ever tried to do chemical research, where Sinaumed made a compound by mixing materials A, B, and C into a test tube?
The ingredients included and the size of the amount cannot be arbitrary because one mistake, Sinaumed, can cause an explosion or poison.
Definition of Proust’s Law
The manufacture of each chemical compound has a composition or arrangement with a fixed number of ratios (ratio) of the constituent materials.
This was stated by a chemist from France named Joseph L. Proust in 1806 with a law that is today known as Proust’s law or law of fixed proportions.
Adapted from Sussex Tech, Proust has argued that compounds of copper carbonate always consist of 5.3 parts copper, 1 part carbon, and four parts oxygen.
The statement made by Proust at that time was very controversial. However, another prominent chemist, John Dalton, supports this statement. Dalton stated that two different carbon compounds were formed from different amounts of oxygen but always had the same composition in each mix.
Adapted from Chemistry LibreText, water compounds are found in various kinds such as drinking water, tap water, rainwater, seawater, and also any water with as much mass as anything; each compound unit always consists of hydrogen and oxygen, which have a mass ratio of 1:8.
This comparison will never change.
From the results of this study, it can be concluded that Proust’s Law is a law that states that a chemical compound consists of several elements which always have the same mass ratio.
Problems example
1. The mass ratio of carbon (C) and oxygen (O) is 3:8. If the carbon that reacts is 1.5 grams, what is the total mass of oxygen that reacted and the group of carbon dioxide that will be formed?
Answer:
Mass of carbon: Mass of oxygen: Mass of carbon dioxide
3: 8: 11
Reaction:
1,5:? 😕
Required mass
8/3×1,5 = 4 gram
The group of carbon dioxide formed
11/3×1,5= 5,5 gram
So the mass of oxygen reacting and carbon dioxide formed is 4 grams and 5.5 grams.
2. The mass ratio of iron and sulfur in iron sulfide compounds is 7:4. What is the mass of sulfur required to form iron sulfide compounds with 21 grams of iron without any reaction residue?
Answer:
Comparison of sulfur and iron x mass of iron
=4/7×21 gram
=12 gram
Then the mass of sulfur needed is 12 grams.
4. Dalton’s Law
Dalton’s law is one of the fundamental laws in chemistry. This law has other names, namely the law of multiple comparisons and the law of multiple comparisons.
The law of multiple comparisons is a law that was coined by John Dalton, a chemist from England. This law is one of the laws that form the basis of the stoichiometry concept. Intrigued by the law of multiple comparisons? Check out the full explanation below!
Definition of Dalton’s Law
According to Dalton, the law of double comparison is two elements that, can form more than one compound when reacted. For example, comparing the mass of one piece, and a combination with another factor with a certain group, is an integer and a simple number.
As for the sound of the law of multiple comparisons that was triggered by John Dalton, it is:
If two types of elements combine to form more than one compound and the mass of one of the elements in the compound is the same. In contrast, the groups of the other aspects are different, then the mass ratio of the other factors present in the mix is a simple integer.
This law was produced by Dalton through experiments conducted and based on the direction of constant comparison that Joseph L. Proust presented.
Dalton believed there was a regularity related to the masses of elements in a compound. The following table is the result of Dalton’s experiment:
An example of applying the law of multiple multiples is the two compounds, sulfur dioxide (SO2) and also sulfur trioxide (SO3).
In one compound molecule, there is one sulfur atom, so both molecules must have the same amount of sulfur mass.
The SO2 molecule has two O atoms, while the SO3 molecule has three O atoms. From this,, it can be concluded that the ratio of O atoms in the two compounds is 2: 3.
Since all O atoms have the same mass, the ratio of the groups of O in the two molecules must equal the atomic ratio, and this ratio (2 : 3) is a ratio using small whole numbers.
Examples of Dalton’s Law Questions
The elements oxygen and phosphorus are reacted to form two types of compounds. In 55 grams of mixture I, there are 31 grams of oxygen. And 71 grams of compound II contains 40 grams of phosphorus.
Does this compound follow Dalton’s law?
Answer:
The mass of phosphorus in compound I = 55-31 = 24
The mass of oxygen in compound II = 71-40 = 31
Thus the mass of oxygen between compounds I and II is the same, namely 1:1. and the masses of phosphorus from compounds I and II are as follows:
24/40 = 4:5
The result is 4:5, which is a simple integer, so the two compounds
This is according to Dalton’s law.
5. The Gay Lussac Law
At the same temperature and pressure, the volume ratio of the reacting gases and the volume of the gas resulting from the reaction is the ratio of simple integers. Joseph Louis Gay Lussac expressed this in the law of volume comparisons. The law of volume comparisons states the volume ratio between the gases involved in a chemical reaction.
Reaction volume comparison experiment
The law of ratios of volumes emerged when Gay Lussac was observing the books of the product and gaseous reactants in the reaction to make water from hydrogen and oxygen. Adapted from Purdue University College of Science, Gay Lussac found that the humidity of the reaction flask did not affect the volume of hydrogen and oxygen gases used in a reaction.
In the experiment, about 199.89 parts by volume of hydrogen were used in the reaction for 100 details by volume of oxygen. From these experiments, the resulting volume ratio with simple integers is 2:1:2.
To prove his discovery, Gay Lussac tried to observe other reactions.
The reaction for the formation of ammonium chloride (NH4Cl) is derived from hydrogen chloride (HCl) and ammonia (NH3). Gay Lussac found that it takes equal volumes of HCl and NH3 to form NH4Cl. That is, the volume ratio of the reactants is 1:1:2. Gay Lussac also discovered that two volumes of carbon monoxide consumed by 1 volume of oxygen would produce two books of carbon dioxide. So, the volume ratio is 2:1:2.
Gay Lussac’s law of volume comparison
Adapted from Chemistry LibreTexts, Gay Lussac’s volume ratio law reads:
“When gases combine at constant temperature and pressure, the volume ratios of the gases involved are always simple integer ratios.”
This means that the ratio between the volumes of the reactant gases and the products can be expressed in integer ratios. This applies to conditions of the same temperature and gas pressure when the reaction occurs.
Problems example
Two liters of chlorine gas reacts with 2 liters of hydrogen gas to produce four hydrogen chloride gas. If 10 liters of chlorine gas has been reacted, how much hydrogen chloride gas will be made?
Answer:
Volume Hidrogen : Volume Klorin : Volume Hidrogen Klorida
2 : 2 : 4
10 : 10 : 20
Then the hydrogen chloride produced from the reaction of 10 liters of water is 20 liters.