Rotary Evaporator: Working Principles, Uses, and Functions – In testing chemical substances, generally, we will carry out several reaction activities.
The reaction activity is carried out, of course, with a specific purpose, such as accelerating reactions, separating substances, dissolving chemicals, evaporating, concentrating, etc.
Evaporation is included in it, which by definition Evaporation or evaporation is the process of changing water molecules into gas.
Yes, the purpose of using an evaporator is to vaporize a solution.
Here’s the full explanation:
Rotary Evaporator: Working Principle, Use and Function
Work principle
Broadly speaking, the Evaporator evaporates the solution, separating the substance from the solvent.
The working principle of a rotary evaporator is to heat the solution in one of the containers (evaporation flask), which is warmed in a water bath, because of the difference in boiling point between the substance and the solvent (e.g., ether, methanol, etc.), the substance with a lower boiling point will evaporate.
The vaporized gas will be trapped by the condenser, which, with the help of cold pressure and temperature, will re-liquid the gas, return to a solution, and be accommodated in a receiving flask.
In the end, two separate liquids will be formed: a concentrated solution and a solvent which generally already contains the dissolved test substance.
Of course, this is a big picture of the working principle of a rotary evaporator; the rest depends on the use and testing in each laboratory.
Usage and Function
The use of a Rotary Evaporator can be very diverse, but the function remains the same, namely separating two solutions with different boiling points.
First Test Example (#1)
For example, if you concentrate, you can use a Rotary Evaporator to evaporate other substances that you did not test.
The concentration of a general solution is done to get a high concentration of the substance we will test.
Because of this, a Rotary Evaporator can evaporate other substances you would not be testing.
Of course, with the difference in boiling points, there will be two separate solutions, and you can test the substance concentrated in the Evaporating Flask.
Second Test Example (#2)
If, in your first example, you focused on the substance being concentrated, this time, you will be testing the vaporized substance.
The working principle is more or less like this:
The substance you are going to test is dissolved in a solvent such as ether, for example, so that the solvent is already bound to the test substance to be evaporated.
By using a water bath and rotating the rotator, the solvent (ether) with varying boiling points can be evaporated and collected in the condenser.
With the help of cold temperatures and air pressure, the gas trapped in the condenser will melt again and be accommodated in the reservoir flask.
So that finally, it forms two solutions, namely the initial solution and the solvent, which already contains the test substance that you will analyze.
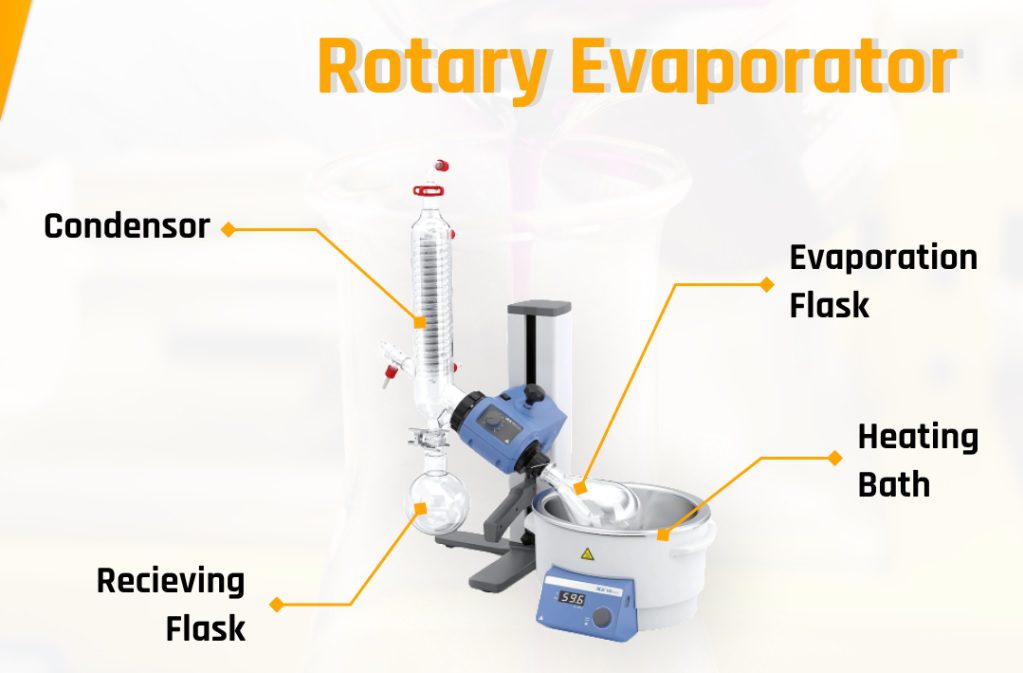
Tool Parts
Seeing the shape that may be quite complex, the Rotary Evaporator consists of several parts.
Here are some of the parts needed to run the tool.
Heating Bath
This section is used to warm the liquid in the evaporation flask.
Instead of using a fired heater which may be dangerous, a water bath can be an alternative solution for providing heat to the solution to be evaporated.
Rotator
The rotator is used to rotate the evaporation flask so that it heats evenly and creates a kinetic movement that makes evaporation more effective.
Without a circular motion, the solution in it may not evaporate completely.
Condenser
This glass device is used to ‘trap’ the air coming out of the evaporating flask with a winding air duct.
In the condenser, manipulation occurs with the addition of cold temperatures and pressure so that the gas, which was originally going to rise to the top, becomes liquid again.
Receiving Flask
This glass tool is used to accommodate the recovered gas from the condenser.
Later this flask contains a solvent that may contain the substance you are testing.
Vacuum Pump
This tool is applied to the condenser, increasing the air pressure, thereby slowing the gas’s movement.
Circulator Chiller
This tool is also applied to the condenser to introduce cold air.